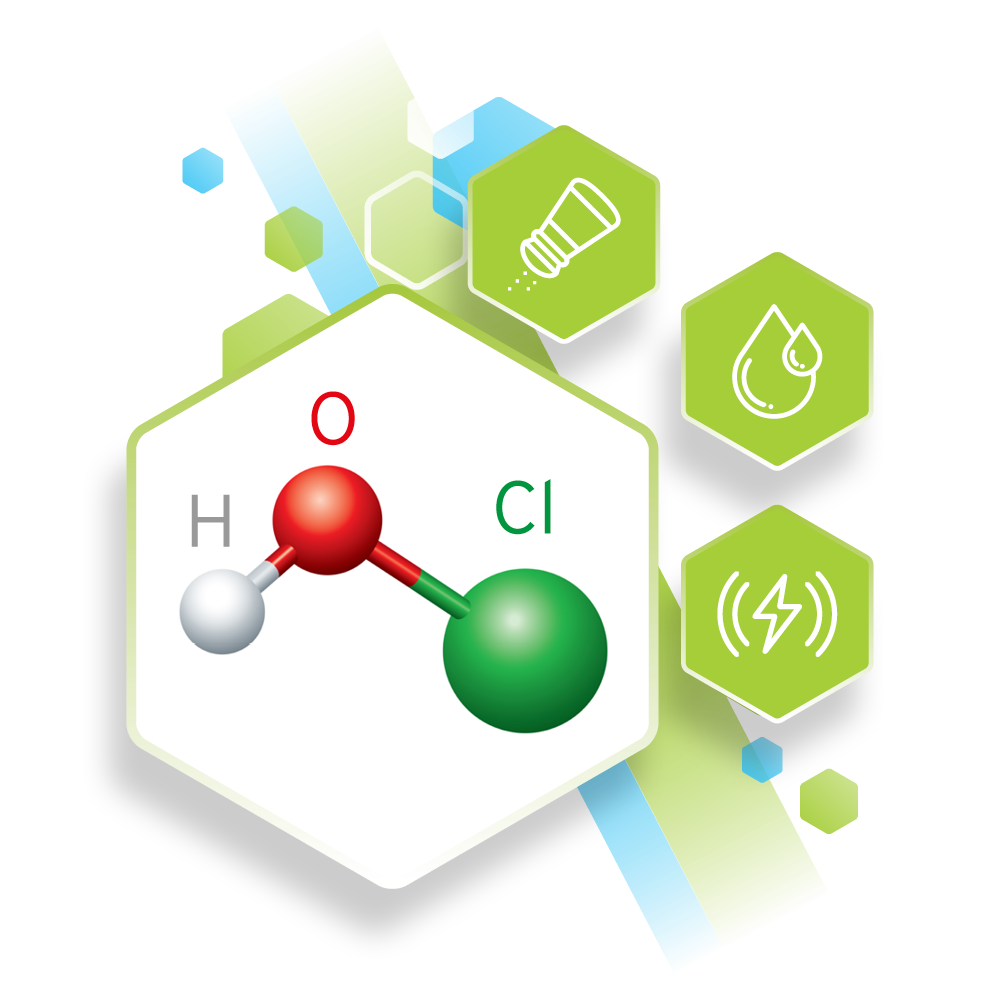
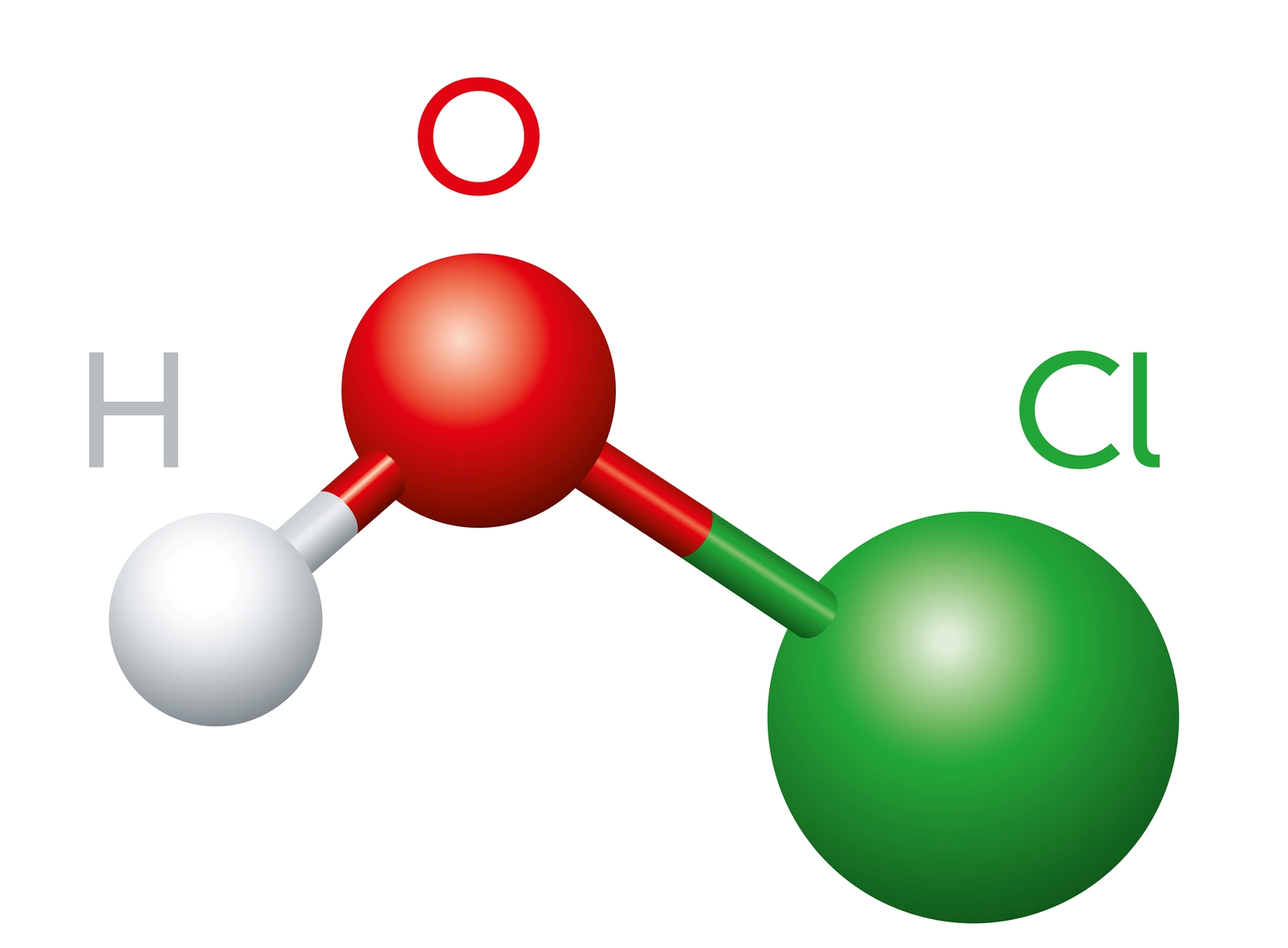
HOCL and Chlorine chemistry
There are two disinfectants associated with chlorine.
HOCl Hypochlorous Acid, which is a very effective disinfectant and OCl- Hypochlorite Ion, is many times less effective than HOCl 2Cl2 + 2H2O = 2HOCl + 2HCl. Chlorine gas when added to water produces Hypochlorous acid plus Hydrochloric Acid. The Hypochlorous Acid then partially disassociates to the Hypochlorite Ion (OCl-): 2HOCl = HOCl + H+ + OCl. The extent of the disassociation depends on the pH of the water (6.5 to 8.5). ECA is produced at a pH of between 5 and 6 making in a powerful and safe disinfectant.
Overall reaction
Various methods have been tried to avoid the use of gaseous chlorine. The most successful is the manufacture of sodium hypochlorite and calcium hypochlorite. Sodium hypochlorite is the main ingredient in bleach. Calcium hypochlorite is primarily used commercially.
Sodium hypochlorite when added to water produces:
NaOCl + H2O = HOCl + OH- + Na+
Sodium hypochlorite plus water produces hypochlorous acid plus hydroxyl ions plus sodium ions. The objective, as with gaseous chlorine, is to add hypochlorous acid to the water. Similar chemistry applies with calcium hypochlorite.
The disadvantage for both sodium hypochlorite and calcium hypochlorite is the need to transfer dangerous chemicals to the point of use or POU. In addition, calcium hypochlorite supports combustion and is a fire hazard.
At Envirocleanse we can produce hypochlorous acid inexpensively and transport our product safely to the POU. There are no transport restrictions, no fire hazards, and we are completely biodegradable. Please refer to our SDS sheet under certifications for further details.
Observations
HOCL from chlorine gas Chlorine gas produces sodium hypochlorite Cl2 + 2NaOH = NaCl +NaClO + H2O Sodium hypochlorite produces hypochlorous acid NaOCl + H2O = HOCl + Na+ + OH- HOCl from ordinary salt Envirocleanse Brine plus electricity produces hypochlorous acid directly 2NaCl + 6H2O + Electricity = 2HOCl + O2 + 4H2 + 2NaOH No harsh chemicals
A History of HOCL
In 1834, the French chemist Antoine Jerome Balard discovered hypochlorous acid (HOCl) when he added a dilute mix of mercury (II) oxide in water to chlorine gas. Additionally, he discovered that HOCl was an effective and safe disinfectant solution.
Later in the 19th century, Michael Faraday was the first scientist to develop a successful technique that generated HOCl from saltwater. The process was called and continues to be known as electrochemical activation.
Although HOCl was a safe, nontoxic, and effective solution to many problems, it was complicated and expensive to maintain a stable version of HOCl with a long shelf life. It was not until recently that scientists were able to produce and maintain stable and cost-effective HOCl and it became suitable for commercial use.

Not all HOCL’s are the same
Envirocleanse-A is the trade name Envirocleanse LLC uses for the hypochlorous acid (HOCL) that we manufacture. HOCL (AKA Anolyte) was invented in 1834 and has been proven to be the safest and most effective disinfectant available. Although there are no poor HOCL products, they are not all the same.
In order to consistently reproduce HOCL that is Ph neutral, has a TDS of 2-3 PPT with FAC of 800 ppm at time of production and have a 12 months shelf life, a defined set of controls and processes, unique to Envirocleanse LLC, must be followed.
Safety Is Paramount
Efficiency and Safety
There’s a reason gloves, face masks, and even safety goggles are essential tools that custodians use when handling bleach, quats, peracetic acid. Bleach solutions emit volatile organic compounds (VOCs), and frequent exposure can cause health complications and render a space less safe to work in.
In terms of efficiency, using bleach alone isn’t enough to make up a robust cleaning program. People would need different products to clean, degrease, disinfect, and sanitize an area. The result is that cleaning routines become more of a chore for custodians and homeowners, not to mention the increased risks of accidents while keeping tabs on the instructions for each product. The effectiveness of bleach can also be affected by several external factors, like heat and light. If it’s not properly stored, bleach will expire sooner than expected.

Hypochlorous acid, on the other hand, not only kills germs more quickly, but notably increases the efficiency of a cleaning and disinfecting routine.
First of all, it doesn’t have a high concentration of toxic compounds. This means that users can handle HOCl solutions without risking bouts of dizziness or burns on their skin.
They don’t have to worry about the long-term health complications that result from continued exposure to chemical cleaners. Secondly, people can work more comfortably without having to juggle multiple formulas as HOCL is an excellent cleaner and bacterial disinfectant.
HOCl is easy to use. It doesn’t involve an endless list of warnings and instructions, so it represents an accessible approach even for those who are unfamiliar with it.

Environmental Outcome
Bleach, quats and peracetic acids have toxic ingredients. The concentration of chemicals varies from product to product. Still, they all release VOCs that spread within indoor spaces whenever these products are used on a surface.
These compounds directly increase air pollution because they remain in the air, and bleach residue is corrosive enough to harm natural resources and the environment upon disposal.
In the same way that HOCl causes no harm to living organisms, it poses no threat to the environment and ensures minimal waste of natural resources.
